www.industry-asia-pacific.com
23
'22
Written on Modified on
PID controllers for the pharmaceutical industry Gefran presents the Multifunction series with advanced features for the traceability of 21 CFR-compliant data
Gefran the Italian multinational group specialised in the design and production of sensors, industrial process control systems, electric drives and automation components has launched the CFR-compliant version of its Multifunction series of multiloop PID controllers, capable of meeting the standards required by the FDA (Food & Drug Administration) under 21 CFR Part 11.

Models 2850T and 3850T CFR, ideal for the measurement, recording and control of production processes, already compliant with the AMS2750 standards for the Aerospace industry and CQI9 standards for the Automotive industry, will now include specific features that are necessary in the pharmaceutical industry, in particular for secondary production plants, such as: sterilisation autoclaves, environmental chambers, filtration systems, freeze dryers and laboratory incubators.
“21 CFR Part 11 is a section of the Code of Federal Regulations that establishes the guidelines of the FDA. In particular, this section refers to pharmaceutical products and medical devices” explains Paolo Buzzi, Controllers & Power Controllers Marketing Manager of Gefran, who continues “Our family of Multifunction products guarantees compliance with the criteria for electronic records and signatures in terms of accuracy, authenticity, and reliability in a digital format. The new CFR-compliant Multifunction products meet these data processing requirements throughout the production cycle”.
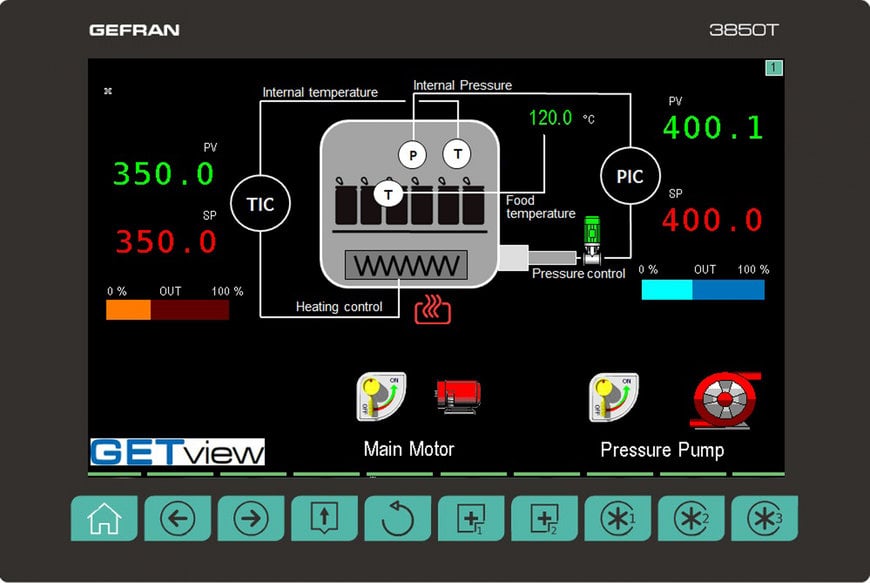
First of all, the controllers 2850T and 3850T CFR enable the creation of an Audit Trail, i.e. the complete history of a process parameter’s creation and changes providing accurate information on who made the changes and when they occurred. This is a necessary condition for 21 CFR purposes, since it ensures the immediate tracking of the processing batch to assess whether any critical issues have occurred.
The recording function also allows process data to be archived in the form of an Electronic Record in combination with an Electronic Signature thus ensuring that changes are tracked and records remain intact. Reports may be exported in standard CSV format or in encrypted form. Furthermore, these devices guarantee the management of passwords with multiple user levels, defining their tasks, permissions and access restrictions, expiration rules and minimum password length, and notification of access attempts - with the possibility of disabling it. Also of particular importance is the launch of an FTP client service, which will allow the controllers to, regularly and independently, send the data record file to a monitoring server.
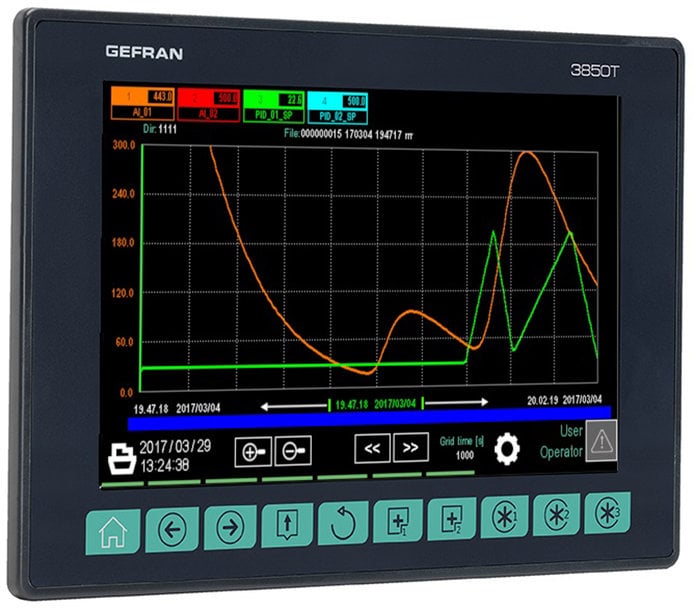
Among the many advantages of the Multifunction series, the temperature and pressure control of a steriliser is a perfect example of its practical excellence. It is particularly relevant the controller’s ability to calculate the F0 coefficient, which measures the lethality of a thermal sterilisation process in relation with time and is useful to evaluate the quality of the operation. The value is entered in the report being generated for each production batch, as information necessary for product traceability.
www.gefran.com

